SCIENCE
Clinical study of XanthigenⓇ
- Subjects: 151 women with obesity,premenopausal and non-diabetic, mean age 36.7 years, average weights 93.8±2.2kg
- Intake: XanthigenⓇ600mg
- Period: 16 weeks
 Increase REE
Increase REE
 Reduction of Body Fat
Reduction of Body Fat
 Reduction of Waist
Reduction of Waist
 Reduction of Visceral fat
Reduction of Visceral fat
 Weight Loss
Weight Loss
Effect of Xanthigen® on body weight in patients with non-alcoholic fatty liver disease (a) and in normal liver fat (b).
Weight decreased in both groups from 6 to 8 weeks after ingestion of Xanthigen®.
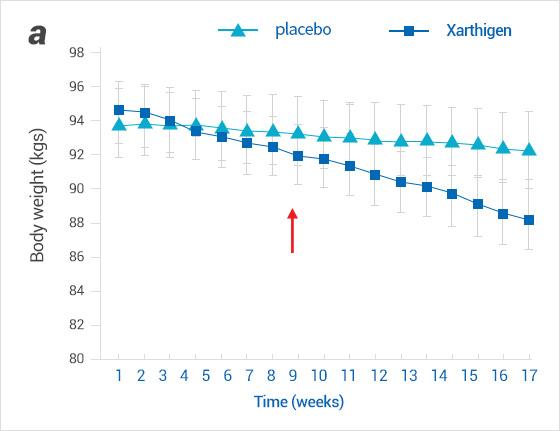
(a) Weights chnage in patients with non-alcoholic fatty liver disease
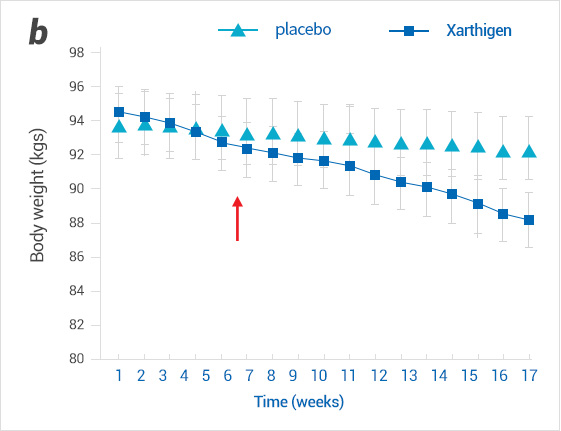
(b)Weights change in normal liver fat group
※Arrows indicate the time(weeks)when the first statistically signficant differences in weight loss between XanthigenⓇ and placebo groups (p<0.05)were noted.
Effects of XanthigenⓇ on resting energy expenditure (REE,kJ/MIN)in obese non-diabetic female voluteers with NAFLD.
- In the test for measuring resting energy expenditure(REE) consumtion, REE was significantly increased compared to the control group when XantigenⓇ 600mg was consumed.
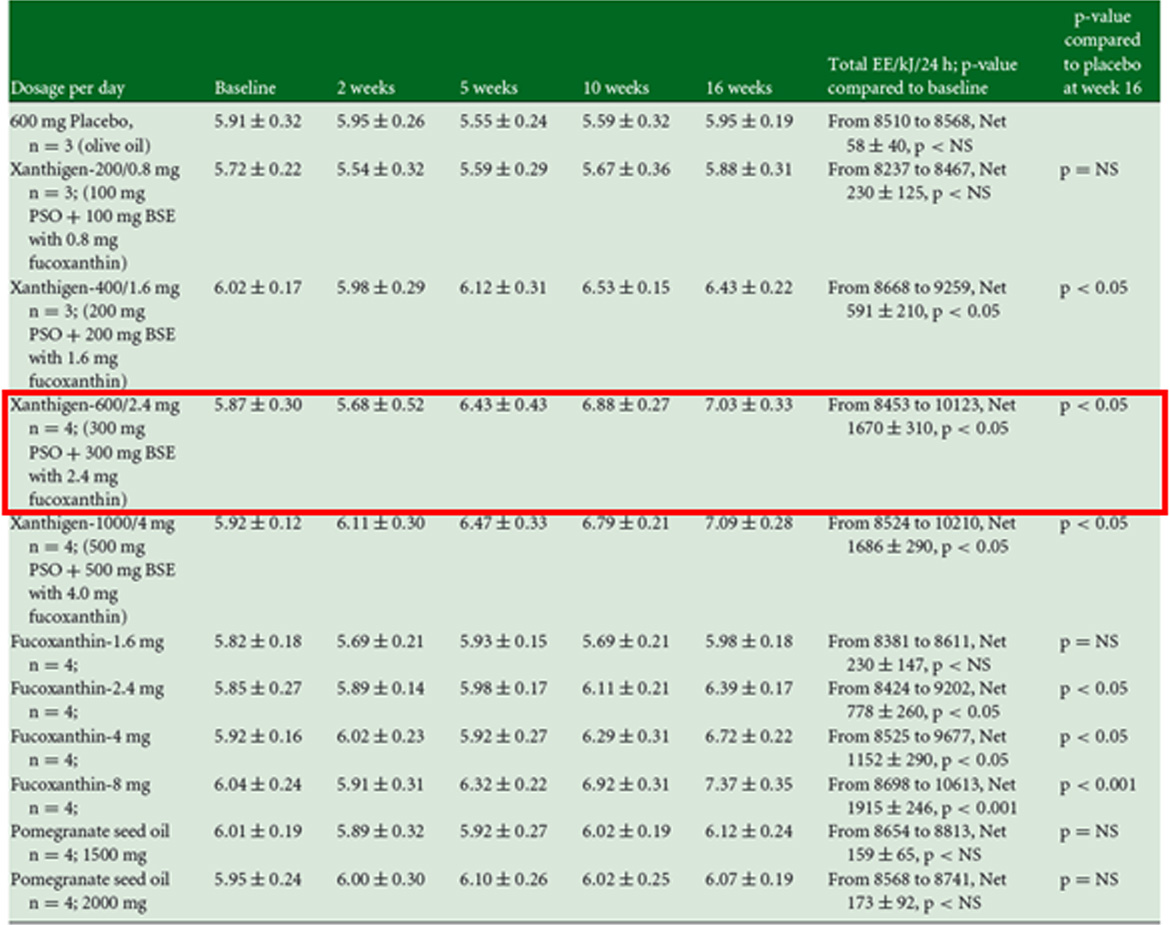
PSO,pomegrante seed oil, BSE, brown seaweed extract; NAFLD, non-alcoholic fatty liver disease.
References
Diabetes, Obesity and Metabolism (2010) 12, 1:72-81 Diabetes,Obesity and Metabolism(2010)12,1:72:81
